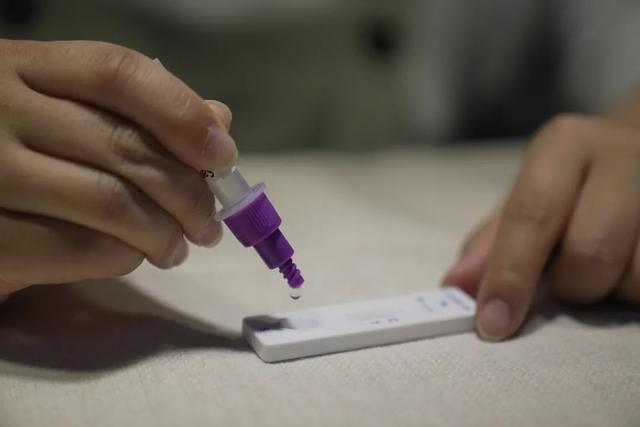
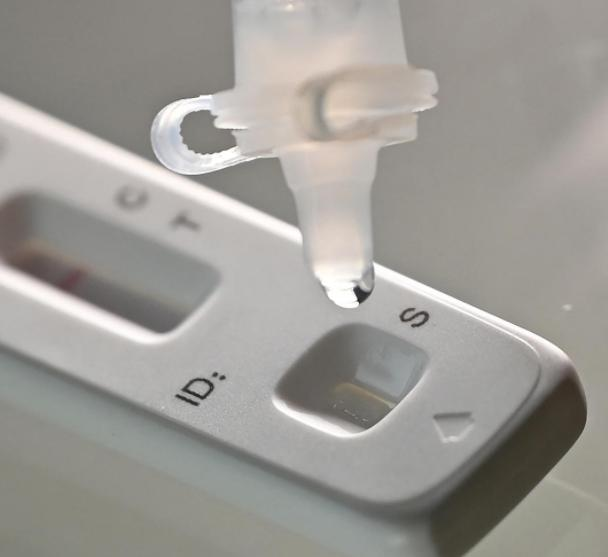
A ranar 12 ga Maristh 2022, daNMPA (SFDA) An ba da sanarwar amincewa da canjin aikace-aikacen don gwajin kai na samfuran antigen na COVID-19 ta Nanjing Vazyme BiotechCo., Ltd, Beijing Jinwofu Bioengineering TechnologyCo., Ltd, Shenzhen Huada Yinyuan Pharmaceutical Technology Co., Ltd, Guangzhou Wondfo Biotech Co., Ltd kumaBeijing Savant Biotechnology Co., Ltd(Huaketai). An ƙaddamar da samfuran gwaji guda biyar na COVID-19 antigen.
A ranar 11 ga Maris, 2022, NHC ta ba da sanarwar cewa, don haɓaka dabarun gwajin Novel Coronavirus da kuma biyan buƙatun rigakafin COVID-19 da sarrafawa, ƙungiyar gama gari ta hanyar haɗin gwiwa da tsarin sarrafawa na Majalisar Jiha ta yanke shawarar ƙara gwajin antigen zuwa gwajin nucleic acid kuma ta sanya “Ka'idojin Aikace-aikacen don Gano Novel Coronavirus Antigenrial)"
Ƙa'idar ta ƙayyadad da yawan jama'a masu dacewa don gwajin antigen:
Na farko, wadanda suka ziyarci cibiyoyin kiwon lafiya na farko kuma suna da alamun cututtuka kamar na numfashi da zazzabi a cikin kwanaki 5 da bayyanar cututtuka;
Na biyu, ma'aikatan lura da keɓancewar keɓe, gami da lura da keɓewar gida, tuntuɓar kusanci da kusanci, lura keɓancewar shigarwa, yanki da ma'aikatan yankin sarrafawa;
Na uku shine mazaunan al'umma waɗanda ke da buƙatar gano kansu na antigen.
Tukwici: Gano Antigen wani muhimmin ƙari ne na gano nucleic acid, amma sakamakon gano kansa na antigen ba za a iya amfani da shi azaman tushen gano kamuwa da cuta ba.
Lokacin aikawa: Maris 22-2022