-

ʻoki ʻoki ʻana - hiki ʻole ke komo
Hoʻopaʻa ʻia ka ʻāpana ʻeha no ka hoʻōla ma hope o ke humuhumu ʻana. Mai ka ʻaoʻao absorption, hiki ke hoʻokaʻawale ʻia e like me ka suture hiki ke hoʻopili ʻia a me ka ʻole. Loaʻa i ka suture non-absorbable ka siliki, Nylon, Polyester, Polypropylene, PVDF, PTFE, Stainless steel a me UHMWPE. He 100% ka nui o ka pulupulu humuhumu kilika mai ka ilo kilika i wiliia. ʻAʻole hiki ke hoʻopaʻa ʻia mai kāna mea. Pono e uhi ʻia ka suture silika i mea e maʻemaʻe ai ke hele ʻana i ka ʻiʻo a i ʻole ka ʻili, a hiki ke coa... -

ʻO ka polyethylene-nui-molecular-kaumaha
ʻO ka polyethylene ultra-high-molecular-weight he ʻāpana o ka polyethylene thermoplastic. ʻIke ʻia hoʻi he polyethylene modulus kiʻekiʻe, he mau kaulahao lōʻihi loa kona, me ka nui molekala ma waena o 3.5 a me 7.5 miliona amu. ʻO ke kaulahao lōʻihi ka hoʻoili ʻana i ka ukana i ka iwi kuamoʻo polymer ma o ka hoʻoikaika ʻana i nā pilina intermolecular. Loaʻa kēia i kahi mea paʻakikī loa, me ka ikaika hopena kiʻekiʻe loa o kekahi thermoplastic i hana ʻia i kēia manawa. WEGO UHWM Nā ʻano UHMW (ultra... -
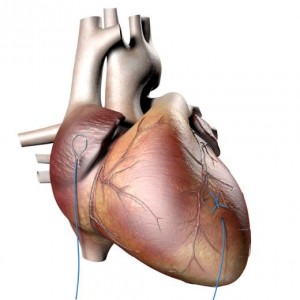
ʻO ka monofilament maʻemaʻe ʻaʻole hiki ke hoʻopili ʻia i nā ʻāpana kila kuhili ʻole - Pacing Wire
Hiki ke hoʻokaʻawale ʻia ʻo Needle i loko o ka lae taper, taper point plus, taper cut, blunt point, Trocar, CC, diamond, reverse cutting, premium cutting reverse, conventional cutting, conventional cutting premium, a spatula e like me kona piko. 1. Nila Lae Taper Ua hana ʻia kēia ʻano kikoʻī e hāʻawi maʻalahi i ke komo ʻana i nā ʻiʻo i manaʻo ʻia. Hoʻokumu ʻia nā paʻi Forceps ma kahi hapalua o ke ala ma waena o ke kiko a me ka hoʻopili ʻana, ʻO ka hoʻonohonoho ʻana i ka mea paʻa o ka nila ma kēia wahi e hāʻawi i ke kūpaʻa hou ma ka n... -
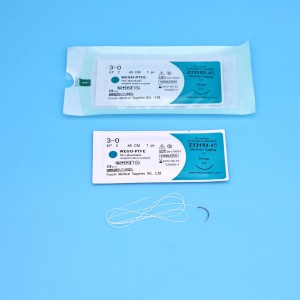
Nā Sutures Polytetrafluoroethylene Steril Non-Absoroable me ka nila ʻole a i ʻole Wego-PTFE
ʻO Wego-PTFE kahi hōʻailona suture PTFE i hana ʻia e Foosin Medical Supplies mai Kina. ʻO Wego-PTFE hoʻokahi wale nō suture i hoʻopaʻa inoa ʻia e Kina SFDA, US FDA a me CE mark. ʻO Wego-PTFE suture he monofilament non-absorbable, sterile surgical suture i haku ʻia me kahi kaula polytetrafluoroethylene, he fluoropolymer synthetic o tetrafluoroethylene. ʻO Wego-PTFE kahi biomaterial kū hoʻokahi no ka mea he inert a me ke kemika non-reactive. Eia kekahi, ʻo ka hana monofilament e pale i ka bacteria ... -

Nā Sutures Polypropylene Sterile Monofilament Non-Absoroble Me ka Needle a i ʻole WEGO-Polypropylene
Polypropylene, non-absorbable monofilament suture, me ka maikaʻi ductility, lōʻihi a me ka paʻa tensile ikaika, a me ka paʻa paʻa kino.
-
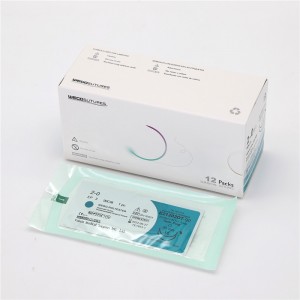
Nā Sutures Polyester ʻAʻole Absoroable Multifilament Me ka Needle a i ʻole WEGO-Polyester
ʻO WEGO-Polyester kahi multifilament synthetic non-absorbable braided i haku ʻia me nā fiber polyester. Hoʻolālā ʻia ke ʻano o ke kaula i hoʻopaʻa ʻia me ke kumu waena i uhi ʻia e kekahi mau kaula liʻiliʻi liʻiliʻi o nā filament polyester.
-
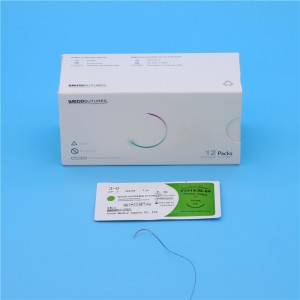
Sterile Multifilament Non-Absoroble Supramid Nylon Sutures me ka ʻole ʻole WEGO-Supramid Nylon
ʻO WEGO-SUPRAMID NYLON suture he synthetic nonabsorbable sterile surgical suture i hana ʻia me ka polyamide, loaʻa i nā hale pseudomonofilament. ʻO SUPRAMID NYLON kahi kumu o ka polyamide.
-
ʻO nā kānana silika multifilament ʻaʻole hiki ke hoʻopili ʻia me ka ʻole a i ʻole ke kui WEGO-Silk
No WEGO-BRAIDED SILK suture, lawe ʻia ke kaula silika mai UK a me Iapana me ka Medical Grade Silicone i uhi ʻia ma ka ʻili.
-
ʻO nā mea hoʻoheheʻe monofilament ʻaʻole hiki ke hoʻopau ʻia ʻo nā nail sutures me ka ʻole a i ʻole ka nila WEGO-Nylon
No WEGO-NYLON, lawe ʻia ke kaula nylon mai USA, UK a me Brazil. ʻO nā mea hoʻolako kaula Nylon like me kēlā mau hōʻailona suture kaulana kaulana.
-

ʻO ka monofilament maʻemaʻe ʻaʻole hiki ke hoʻopili ʻia me ke kila kila ʻole me ka nila ʻole a i ʻole WEGO-Stainless Steel.
ʻO ka hiku kila kuhili ʻole he ʻoki ʻoki kino ʻole nonabsorbable i haku ʻia me 316l kila kila. ʻO ka ʻokiʻoki ʻana i ke kila kuhili ʻole he monofilament kila kūpaʻa ʻole i hiki ke hoʻopaʻa ʻia a ʻōwili ʻia paha (axial). Hoʻokō ʻia ka hiku kila kuhili ʻole i nā koi a pau i hoʻokumu ʻia e ka United States Pharmacopoeia (USP) no nā ʻoki ʻoki ʻoki ʻole. Hoʻopaʻa inoa ʻia hoʻi ka humuhumu kila kuhili ʻole me ka helu B&S gauge.
-
Nā Sutures Steril Monofilament Non-Absoroble Polyvinylidene fluoride me ka nila ʻole a i ʻole WEGO-PVDF
ʻO WEGO PVDF kahi koho ʻē aʻe i ka polypropylene ma ke ʻano he monofilament vascular suture ma muli o kāna mau waiwai physicochemical maikaʻi, maʻalahi o ka lawelawe ʻana, a me kona biocompatibility maikaʻi.
-
Nā Suture Polytetrafluoroethylene Sterile Monofilament Non-Absoroble me ka nila ʻole a i ʻole WEGO-PTFE
ʻO WEGO PTFE he monofilament, synthetic, non-absorbable surgical suture i haku ʻia me 100% polytetrafluoroethylene me ka ʻole o nā mea hoʻohui.














